| The Second Huge Stem Cell Breakthrough in a Week | |||
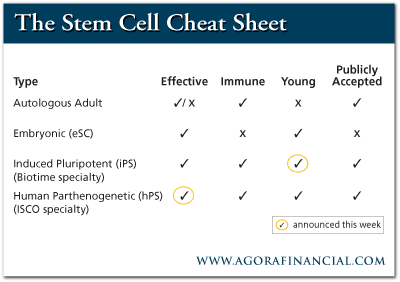
I'll explain the ISCO developments in some detail. A reader, by the way, recently suggested that I write the Stem Cells for Dummies book for those who are confused by this complex field. While this is not a book, it should help those of you who really want to understand the state of this critical science. As a primer, it's vital that you're up to speed on the four different types of stem cells (SCs): Adult Autologous SCs - SCs taken from donor bone marrow and fat tissue, cultured and re-administered to the donor for therapeutic purposes. These solve the immune and ethical problem but do not compare in power to the other three types, nor are they "young," meaning maximum telomere length. Embryonic SCs - These cells are taken from embryos and are therefore ethically objectionable to many people. They have efficacy and they are young but they provoke an immune response. Induced Pluripotent SCs - These are SCs that are created by genetically engineering normal adult cells to revert to SC status. They have efficacy and they solve the immune problem because they are the donor's own cells. Last week, BioTime proved that they can also be made "young." Parthenogenic SC - These SCs come from unfertilized oocytes, the cells that, if allowed to develop, would become ovum. They are, by definition, young. They have no ethical problems because they are not embryonic. They solve the immune problem through the creation of ISCO's HLA typed cell bank. Last week, ISCO also announced evidence that they have efficacy. Once you've got those covered it's equally important to take a look at what each treatment is capable of...
The above chart, and corresponding announcements are simply fantastic news - notably for iPS and hPS. I updated you on BioTime's breakthrough regarding iPS last week in a special alert - but soon after BioTime's announcement there was another huge happening. Today I want to catch you up on this important news... Unlike BioTime's breakthrough, which was covered by The Wall Street Journal, The New York Times and other big media, ISCO's breakthrough announcement was, basically, ignored. The reasons are twofold. First, ISCO may be the most insouciant, understated biotech in business today. Some companies are quick to trumpet their accomplishments. ISCO, on the other hand, is incredibly and systematically low-key in its public statements. Company announcements are often cautious to the point of secretiveness. I'm convinced that the company's stock price would be much higher now if this were not the case. Secondly, I admit the significance of last week's announcement is particularly well obscured. Therefore, let me read between the lines of this press release. On the surface, the press release is about the beginning of ISCO's "Second Pre-Clinical Phase of Testing Retinal Pigment Epithelium (RPE) Derived From Human Parthenogenetic Stem Cells for Treatment of Retinal Diseases." So why is this a big deal? It is a big deal because of one sentence in the press release. "Encouraging data from animal models have shown that visual degradation caused by AMD can be slowed through the transplantation of RPE." In other words, ISCO has evidence that its nonembryonic parthenogenic RPE cells halt the advance of age-related macular degeneration. These cells prevent blindness just as RPE cells from cadavers and embryonic sources do. In essence, this is the same kind of breakthrough that BioTime just announced, but from a different angle. Here's how the story played out for BioTime... Scientists have long known that embryonic stem cells have the ability to repair damaged tissues in animals. Scientists also knew that induced pluripotent stem (iPS) cells made from adult cells (not embryos) would have the same ability. This is important because iPS cells don't come with the ethical problems that embryonic cells do. Moreover, because iPS cells can be made using the donors' own cells, they would cause no immune system rejection problems if returned to the donor. The rejection problem could likely be treated using immune system suppressants, just as is done in the case of organ transplants. Still, immune suppressants have many unwanted side effect. Donor iPS cells would clearly be a superior solution. Scientists were excited because iPS cells offered the hope that youthful iPS cells could be created using a patient's own cells. Then, you could have as many of your own rejuvenated cells as you needed to fix aged and damaged tissues, all without immune reaction. The monkey wrench in this vision came when some scientists created iPS cells from donor cells. They found that the effective age of the new stem cells was the same as the donor cells. This was a big potential problem. They believed that iPS heart cells from a 65-year-old donor who had suffered a heart attack would be effectively 65 years old. There would be little point in transplanting old cells into a heart suffering from aging-related illness. West, however, proved that he can reverse the clock of aging in iPS cells so that the 65-year-old woman with heart disease could use her own iPS cells that are as healthy as a newborn baby's. This is huge. Basically, ISCO scientists have just proven that they have the same ability vis-a-vis parthenogenic cells. There has never been any question that hPS cells are young. This is because they are derived from unfertilized ovum, oocytes. Like iPS cells, hPS cells also solve the ethical problems associated with embryonic stem cells. They are not embryonic cells and, unlike iPS, probably can't even be used to create human clones. Moreover, the parthenogenic technology has the promise of solving the rejection problem, as do iPS cells. Parthenogenic stem cells have only half the DNA of normal cells. They are parthenotes, cells whose double helix of DNA has unzipped in preparation for the possibility of fertilization. ISCO has figured out how to use this feature to "hack" the immune system problem. The reason is that immune rejection or acceptance is determined by human leukocyte antigen (HLA) matching. This is too complicated a subject to deal with sufficiently here, but you can think of it this way: Acceptance or rejection of a transplant organ or cell depends on the body's reaction to a number of genetic "points," or antigens in the transplanted tissue. With good matching of these points, little or no immune suppression is necessary. If most do not match, there will be massive rejection and even the most aggressive immune suppression may not work. For simple mathematical reasons, the odds against comprehensive matching of HLA points increase geometrically with each additional point. Therefore, the difficulty of immune matching hPS cells, with half the DNA and HLA points of normal cells, is far, far less than half the difficulty of matching a normal cell. In fact, it is possible that only 50 hPS cell lines will immune match 95% of the human race. Think of these 50 (or more) cell lines as complex versions of blood types. ISCO is in the process of building a cell bank of hPS cells that could solve the rejection problem, delivering the immune benefits of iPS cells, but from another angle. ISCO has several lines now that each immune match over 15 million Americans. Once gathered, these immortal cell lines never have to be regathered. Even unmatched, by the way, hPS cells with half the HLA points are probably superior to normal embryonic cell therapies in the immune suppression department. Once this cell bank is constructed, however, ISCO expects to solve most of the same immune rejection problems that iPS cells solve. Additionally, as I've said, they are young cells like BioTime's iPS cells. Only one piece of the puzzle was unverified. We did not have proof until this last ISCO study was completed that hPSCs actually work. I believed they would. ISCO scientists, obviously, believed they would, but we didn't have clinical evidence. It was an extrapolation of scientific principles. When making projections, we always make these sorts of assumptions. If ISCO and I had been wrong, however, ISCO would be collapsing and its stock value near zero. And I would be... embarrassed, to put it mildly. So my thanks go to all the scientists at ISCO, especially Dr. Elena Revazova, who made the early parthenogenic breakthroughs in Russia. Anyway, we now have clinical evidence that hPS cells work in animals, just as embryonic cells and iPS cells do. Increasingly, by the way, it has become obvious that embryonic stem cells are functionally obsolete in the field of regenerative medicine. There has been an unusual amount of resistance to this fact in the scientific press. I'm half-convinced, in fact, that a lot of journalists are still vested in discrediting President Bush's insistence that a superior solution to embryonic stem cells could be found. Embryonic stem cells work fine, of course, for actual human reproduction. I personally hope they never become obsolete there. iPS and hPS cells, however, are the superior platforms for most therapeutic uses. The iPS platform may always be the most attractive high-end stem cell (SC) strategy, but ISCO's hPS cell therapies will have important advantages in many cases. For instance, let's say you have an accident and need SC treatment immediately for spinal cord or organ injuries. Unless you have already had your cells transformed into iPS cells and potentiated to become all the types you are likely to need in emergencies, your only real option would be hPS cell therapy. ISCO's platform will also have a cost advantage. ISCO's ability to mass-produce and store off-the-shelf treatments, stem cells programmed to cure specific conditions without immune rejection, guarantees the company a huge market niche. Induced pluripotent SC technologies will likely keep the high-end clinical market, but hPS cells will likely be the Wal-Mart of SC therapies. While not as glamorous as the high end, Wal-Mart has made early investors rich. ISCO's advantages should do the same thing. For example, consider the RPE cells ISCO is bringing to market. These cells are based on 10 years of research, primarily at Advanced Cell Technology (ACTC: OTCBB). Ten years ago, age-related macular degeneration (AMD) looked like the perfect target for eSC therapies. It is known that RPE cells from cadavers can stop or reverse AMD. The cost of gathering enough cadaver cells for one patient, however, runs from $10,000-15,000. Moreover, there are simply not enough cadaver cells for a fraction of the 2.5 million Americans with the disease. ACT estimates the worth of the market for replacement RPE at $2.5 billion. ISCO's chairman Ken Aldrich, in keeping with his aforementioned restraint, says $1 billion. Retinas, unlike corneas, are rich with capillaries. This means immune reaction is a particularly serious issue. When ISCO gets to market in two or three years, I project it should have most or all of the cell lines it needs to deal with immune rejection. Moreover, it has taken the technology a step further. Whereas ACT proposes therapy with RPE cells, ISCO is growing them in a matrix that can be applied surgically. My prediction is that these matrices will not only stop AMD, they will reverse it. If ISCO had a theme song, it would probably be Irving Berlin's "Anything You Can Do." We'll see, of course, but all that's left to prove ISCO as a transformational stock for the ages is human trials. They could be completed in as little as two years. Patrick Cox for The Daily Reckoning Joel's Note: It's difficult not to get excited about the kinds of breakthrough technology Patrick regularly uncovers for his readers. A staunch believer in human ingenuity, Patrick is the kind of fellow who sees a silver lining for (almost) every cloud. It's refreshing, yes...but it can also be immensely profitable, too. Imagine having invested in some of the past breakthrough technologies - the automobile, for example - before they changed the course of history. Now we're talking about the kind of fortunes that can last over many generations. |
Stem Cell Health is simple and natural. Embrionic Stem Cells are Controversial Conversation. Consequently Stem Cells are hot political news. We are interested only in what everyone agrees about -- no bull here.
Wednesday, March 24, 2010
The Second Huge Stem Cell Breakthrough in a Week
Subscribe to:
Post Comments (Atom)

No comments:
Post a Comment